Neonatal Intensive Care Unit (NICU)
Focusing on the Most Vulnerable Patients
Home / Neo-Intensiv
Neo-Intensiv
From the beginning, we have kept neonatal patients close to our hearts, and Masimo remains dedicated to improving their care through industry-leading solutions designed to ensure that even the youngest patients have bright futures.
Masimo SET® Measure-through Motion and Low Perfusion™ Pulse Oximetry
Masimo SET® Measure-through Motion and Low Perfusion™ Pulse Oximetry
Masimo’s breakthrough technology, Signal Extraction Technology® (SET®), overcame the limitations of conventional pulse oximetry with the ability to measure through motion and low perfusion. Masimo SET® is able to recognize that both arterial and venous blood can move, using parallel signal processing engines to separate the true arterial signal from sources of noise, including the venous signal. Two separate studies found that Masimo SET® pulse oximeters detected approximately 10 times more true events than other “Next Generation” pulse oximeters studied.1,2 Additionally, in another study comparing the ability of three pulse oximetry technologies to detect hypoxic events, Masimo SET® pulse oximetry demonstrated the highest sensitivity and specificity during induced conditions of motion and low perfusion.3
Performance During Motion and Low Perfusion3
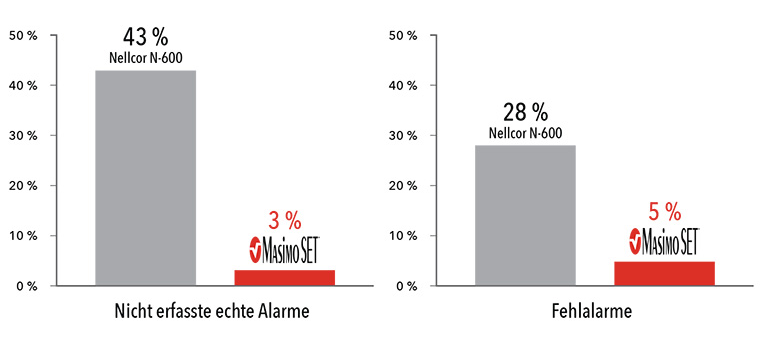
Masimo SET® had 3% missed true alarms and 5% false alarms versus 43% and 28%, respectively, using competitor technology.
Results shown are calculated by combining sensitivity and specificity outcomes of machine-generated and volunteer-generated motion.
Reducing Retinopathy of Prematurity (ROP)
Reducing Retinopathy of Prematurity (ROP)
Premature infants in neonatal intensive care units are routinely administered supplemental oxygen to help preserve vital organ function. However, too much oxygen administration, which can lead to hyperoxia, can increase the risk of severe eye damage from retinopathy of prematurity (ROP).4 Clinicians use pulse oximetry to help manage the administration of oxygen, but unreliable pulse oximetry measurements can result in over administration of oxygen and subsequent ROP.
In two NICU settings, Masimo SET® Measure-through Motion and Low Perfusion pulse oximetry, coupled with changes in clinical practice, showed significantly reduced rates of severe retinopathy of prematurity (ROP) and decreased the need for laser treatment to 0%.5,6
Severe Retinopathy of Prematurity Rate5
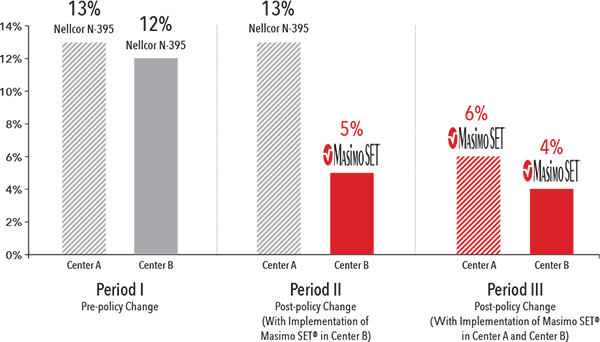
In a study of 571 inborn infants (<1250 g), a change in clinical practice combined with the use of Masimo SET® pulse oximetry, but not without it, led to significant reductions in severe ROP and need for laser therapy.5
Rates of Retinopathy of Prematurity in Very Low Birth Weight Infants6
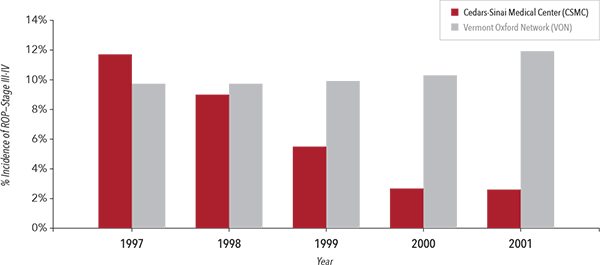
In another study, following the implementation of Masimo SET® with a new protocol in a single tertiary neonatal center at Cedars-Sinai Medical Center (CSMC), the incidence of severe ROP decreased over a five-year period from 12.5% to 2.5% in very low birth weight infants.6
Acquired Methemoglobinemia
Acquired Methemoglobinemia
Methemoglobinemia is a blood disorder characterized by an abnormal amount of methemoglobin – a form of hemoglobin unable to effectively bind to oxygen – in the blood. Many drugs commonly used in hospitals — such as lidocaine, benzocaine, dapsone, and nitrates — may cause a dangerous reaction known as acquired methemoglobinemia.7 Inhaled nitric oxide (iNO) therapy, and even topical anesthetics containing benzocaine or prilocaine, can cause elevated levels of methemoglobin in neonates and infants.8,9
Masimo Pulse CO-Oximetry provides a method of noninvasively and continuously measuring methemoglobin, SpMet®. Real-time SpMet monitoring may help clinicians intervene quickly and appropriately in cases involving elevated methemoglobin levels.10
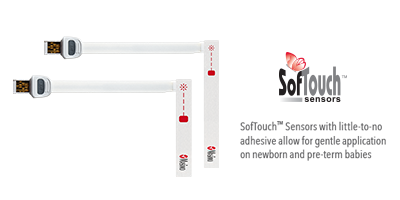
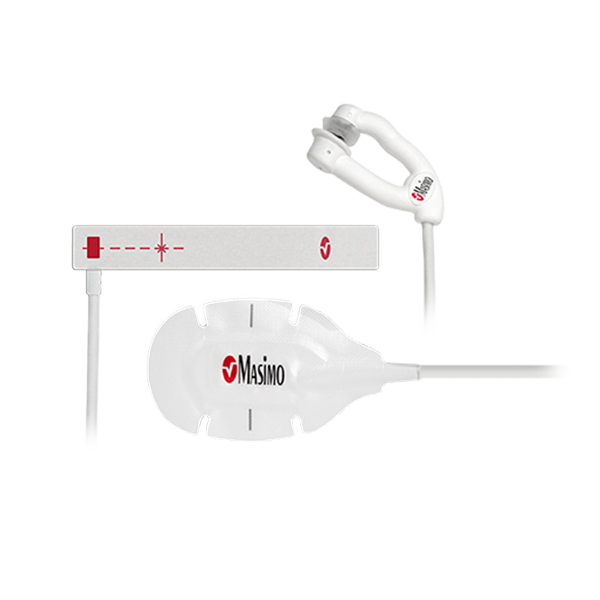
Sensors Designed for Neonatal Patients
Capnography Solutions
Capnography Solutions
Masimo offers a portfolio of sidestream capnography solutions to meet the challenges of ventilation monitoring in the NICU. Sidestream CO2 monitoring works with a full family of NomoLine™ sampling lines, airway adapter sets, and cannulas for use in a variety of clinical scenarios, including intubated and non-intubated patients in both low and high humidity applications. NomoLine technology is designed for low-flow applications, with a very low sampling rate of 50 ml/min, supporting use on patients with low tidal volumes and high breath rates, common characteristics of neonatal patients. Masimo capnography seamlessly integrates with Masimo SET® pulse oximetry and other key physiologic parameters, providing clinicians with greater visibility to a patient’s oxygenation, ventilation, and respiratory status.
Measurements
Measurements
Products
References:
- 1.
Hay WW. Reliability of conventional and new oximetry in neonatal patients. J of Perinatol, 2002;22:360-36.
- 2.
Barker SJ. "Motion-Resistant" Pulse Oximetry: A comparison of new and old models. Anesth Analg. 2002;95(4):967-72.
- 3.
Shah et al. J Clin Anesth. 2012;24(5):385-91.
- 4.
Saugstad et al. Journal of Perinatal Medicine. May 2006.
- 5.
Castillo et al. Prevention of retinopathy of prematurity in preterm infants through changes in clinical practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- 6.
Chow et al. Can changes in clinical practice decrease the incidence of sever retinopathy of prematurity in very low birth weight infants? Pediatrics. 2003;111(2):339-345.
- 7.
Ash-Bernal R et al. Medicine (Baltimore). 2004 Sep. 83(5):265-73.
- 8.
Riou Y et al. Pediatric Research (1998) 43, 295-295.
- 9.
U.S. Food & Drug, Consumer Updates, Benzocaine and Babies: Not a Good Mix.
- 10.
Annabi EH et al. Anesth Analg. 2009 Mar;108(3):898-9.
* The use of the trademark PATIENT SAFETYNET is under license from University Health System Consortium.
RESOURCES
Rad-97 with NomoLine capnography is not licensed for sale in Canada.
SpMet monitoring is not intended to replace laboratory blood testing. Blood samples should be analyzed by laboratory instruments prior to clinical decision making.
Vorsicht: Laut US-amerikanischem Bundesgesetz darf dieses Gerät nur von einem Arzt oder auf Anweisung eines Arztes verkauft werden. Vollständige Verschreibungsinformationen einschließlich Indikationen, Gegenanzeigen, Warnungen und Vorsichtsmaßnahmen finden Sie in der Gebrauchsanweisung.
PLCO-001785/PLM-11086B-0318